Last Updated: July 18, 2023
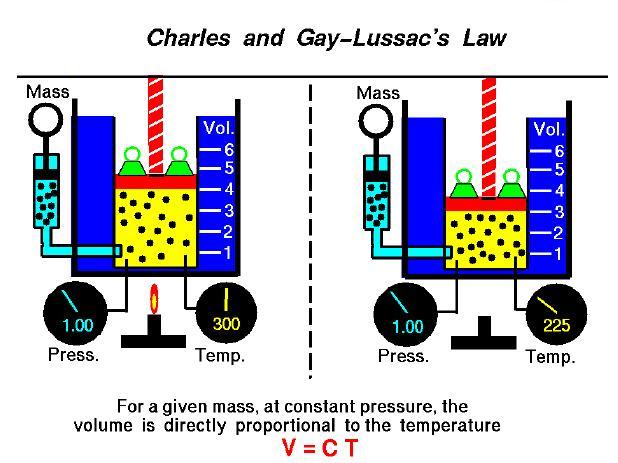
Charles’ Law studies the gas volume and how temperature affects the gas pressure and volume. This law was developed by a French scientist, mathematician, balloonist, and inventor, Jacques Alexander Charles. In 1787, he formulated a law by understanding how temperature affects gas volume.
If we refer back to Boyle’s Law, it states that if the temperature remains constant, the volume of gas is proportional to the absolute pressure. However, Boyle did not concern himself much with changing temperature. And this was the main goal of Jacques Charles.
You must be thinking about how does Charles’ law applies to scuba diving though? This law shows why an increase in temperature leads to an increase in pressure inside the scuba tank.

To understand it in more detail, let’s look at what Charles’ law states.
The Formula
The mathematical representation of Charles’ Law is:
P1 x V1 / T1 = P2 x V2 / T2
Here, the temperature is denoted by T, pressure by P, and volume by V. 1 is the initial volume and temperature, and 2 is the final volume and temperature.
So he basically added the T variable to the Boyle’s law and the formula is also called the General Gas Law.
Charles’ law explains that if you take a balloon, fill it with air and then increase the air’s temperature; you will see an increase in the air’s volume, which will lead to expansion of the balloon. This occurs since the air molecules heat up and move rapidly. Similarly, if the balloon is cooled in a freezer, the air volume will decrease and result in partial deflation of the balloon.
When you heat a scuba tank of 3000 psi, the pressure inside the tank will increase since the air volume will remain constant inside the tank.
How Charles’ Law Applies to Scuba Diving?
So how Charles’ law applies to scuba diving through the above explanation? It warns divers of the repercussions of leaving a scuba tank outside in the hot sun. This is also why it is against professional advice to leave or transport tanks in a hot car. When the pressure increases, the tank could explode.
It is necessary to store the tanks in the right manner so that no mishaps occur due to overheating. The Law shows how the pressure inside the tank decreases or increases depending on the temperature. It also shows why the scuba tank can get hot when you fill it with compressed air.
How Effective is Charles’ Law For Scuba Diving?
Charles’ Law explains a very simple phenomenon related to scuba tanks, but also warns of impending danger. Although it is derived after watching the effects of pressure inside a balloon, however, the impact of a bursting scuba tank will be much more dangerous than that of a balloon.
You can see the importance and effectiveness of this law in the several safety measures that scuba tanks have today. Some dive shops overfill their scuba tanks so that divers do not get less gas when they are underwater. However, these overfilled tanks can cause hazards if left out in the open.
Final Word
You will find that modern scuba tanks with safety clips that allow pressure to escape by splitting. This prevents explosion to a great extent. Charles’ law may not be as effective as Boyle’s, but it helps you understand how to prevent mishaps related to scuba tanks.

My unbounded love for the oceans and everything it has to offer motivated me to pursue my passion and become a professional scuba diving instructor.
I keep reading, exploring, and learning more about scuba diving and the underwater world all the time, so I’m excited to share my knowledge with fellow scuba enthusiasts and hopefully contribute a little to your development as a diver. I want people to fall in love with the oceans with as much passion as I have. Read more about me here.
